Recently, a research team led by Prof. Liping Qiu from the Hangzhou Institute of Medicine of Chinese Academy of Sciences has developed DNA nanomachines with different lipid modifications, enabling their targeted localization in different domains of the cell membrane and regulation of the partition distribution of target membrane proteins (PTK7, CD45). This research explores the biological effects of protein membrane distribution in cancer cell migration and T cell activation processes.
The study was published in Journal of the American Chemical Society.
The non-uniform distribution of molecules such as lipids and proteins in the cell membrane leads to the formation of complex structural domains (also known as structural domains), such as lipid ordered (Lo) domains (also known as lipid raft domains) and lipid disordered (Ld) domains. The dynamic reorganization of cell membrane domains and the partitioning of integral membrane proteins play important roles in life processes such as cell signal transduction. The dynamic manipulation of protein distribution in cell membrane domains is of great significance in decoding cellular life processes. Genetic modification strategies can be used to construct fusion proteins with specific localization in the cell membrane, but it is difficult to avoid changes in protein structure, expression levels, and potential functional impacts. Therefore, the development of regulatory methods for protein membrane distribution under non-genetic modification conditions is essential but highly challenging.
In this study, the researchers constructed three base-modified cholesterol (Tcho3) or tocopherol (Ttcp3) DNA tetrahedra probes to achieve their targeted binding to different domains of the cell membrane. Furthermore, they constructed DNA nanomachines by using toehold-mediated strand displacement reactions to introduce protein recognition ligands (nucleic acid aptamers or antibodies). The DNA nanomachines can dynamically control the distribution of target membrane proteins (such as the tumor marker protein PTK7 or the immune protein CD45) in Lo and Ld domains and explore the relevant biological effects.
In the real cell membrane and giant plasma membrane vesicles (GPMVs) system derived from cells, with commercial cell membrane Lo domain dye CTB and Ld domain dye F-DiI as references, the researchers used the fluorescence colocalization technique combined with relevant quantitative analysis to examine the targeting performance of the two DNA tetrahedron probes to different cell membrane domains. The results indicated that Tcho3 tends to bind to the cell membrane Lo domain, while Ttcp3 tends to bind to the cell membrane Ld domain.
Subsequently, to target the membrane protein PTK7, the researchers selected the nucleic acid aptamer sgc8c for its recognition and binding. They used Toehold-mediated strand displacement reactions to assemble DNA nanomachines (Ap-Tcho3 and Ap-Ttcp3) and examined and verified the successful assembly of DNA nanomachines at the interface of solution and cell membranes. They also examined the relative content of PTK7 by extracting the membrane lipid raft structures after the action of DNA nanomachines. The results indicated that Ap-Tcho3 successfully achieves PTK7 localization regulation in the Lo domain of the cell membrane, while Ap-Ttcp3 regulates PTK7 more towards the Ld domain of the cell membrane.
The researchers investigated the potential impact of membrane distribution regulation on the biological function of PTK7. By using cell scratch assays and trajectory tracking experiments, they examined the effect of PTK7 distribution regulation on the migration ability of colon cancer cells HCT116. The researchers found that the accumulation of PTK7 in the Lo domain promotes tumor cell migration, while conversely, PTK7 in the Ld domain inhibits tumor cell migration. They also found that the regulation of PTK7 to the Lo domain promoted cell endocytosis, while regulation to the Ld domain inhibited cell endocytosis. They speculated that the differential internalization mediated by membrane partition distribution was the main source of PTK7's effect on tumor cell migration.
Furthermore, the researchers introduced CD45 antibodies to constructed DNA nanomachines based on the modular properties of DNA nanomachines, verifying their regulatory ability on the membrane partition distribution of CD45. They also evaluated the impact of CD45 partition distribution on T cell immune activation by using various T cell activation parameters. The experimental results showed that the dynamic allocation of CD45 between the Lo and Ld domains of the cell membrane can achieve modulation of T cell activation.
The DNA nanomachines hold promise as a universal technology platform for controlling protein partition distribution on the cell membrane, providing new methods and dimensions for the precise regulation of complex cellular signaling networks.
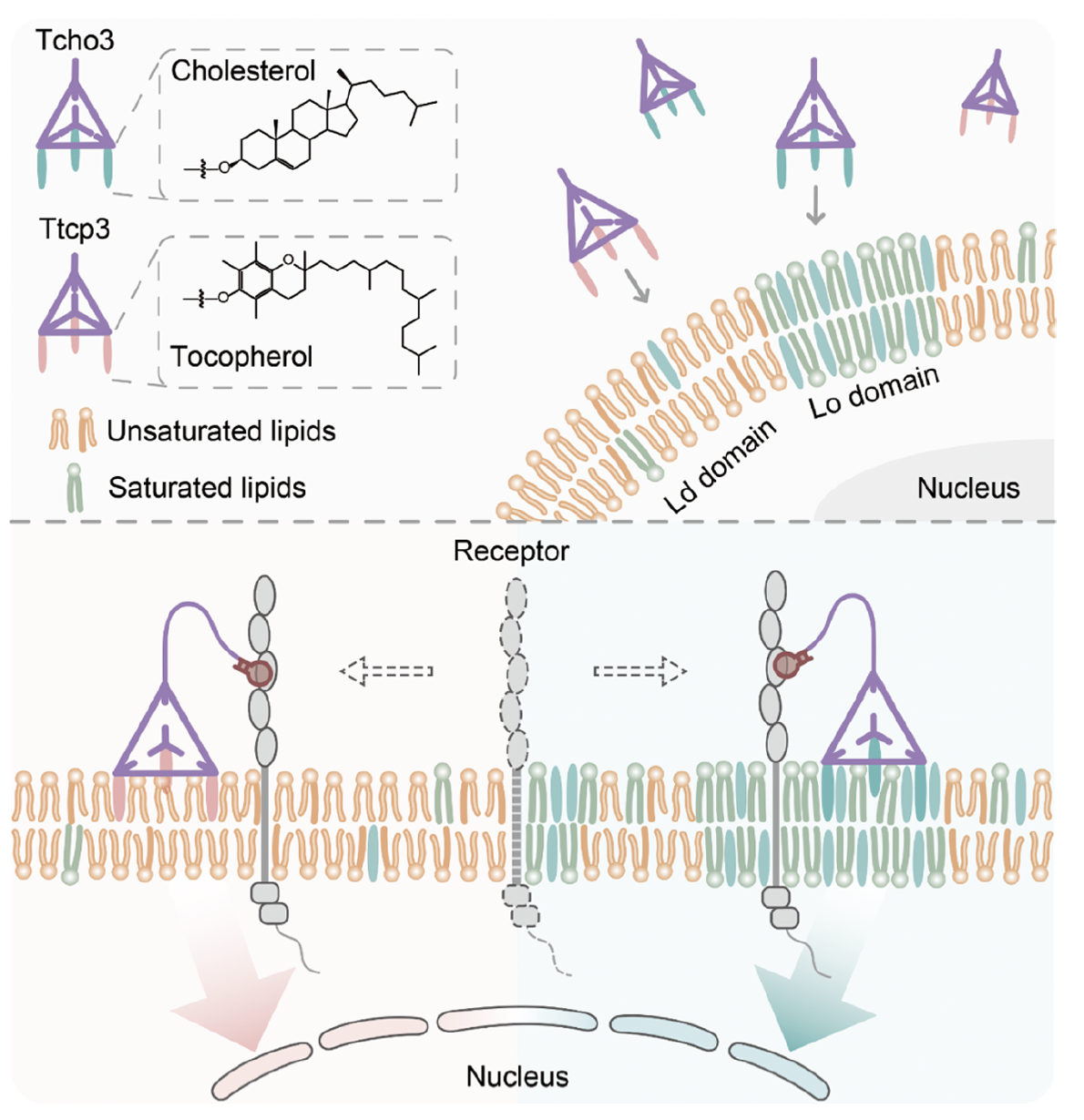
Schematic diagram of DNA nanomachine regulating the partitioning distribution of proteins in the cell membrane



