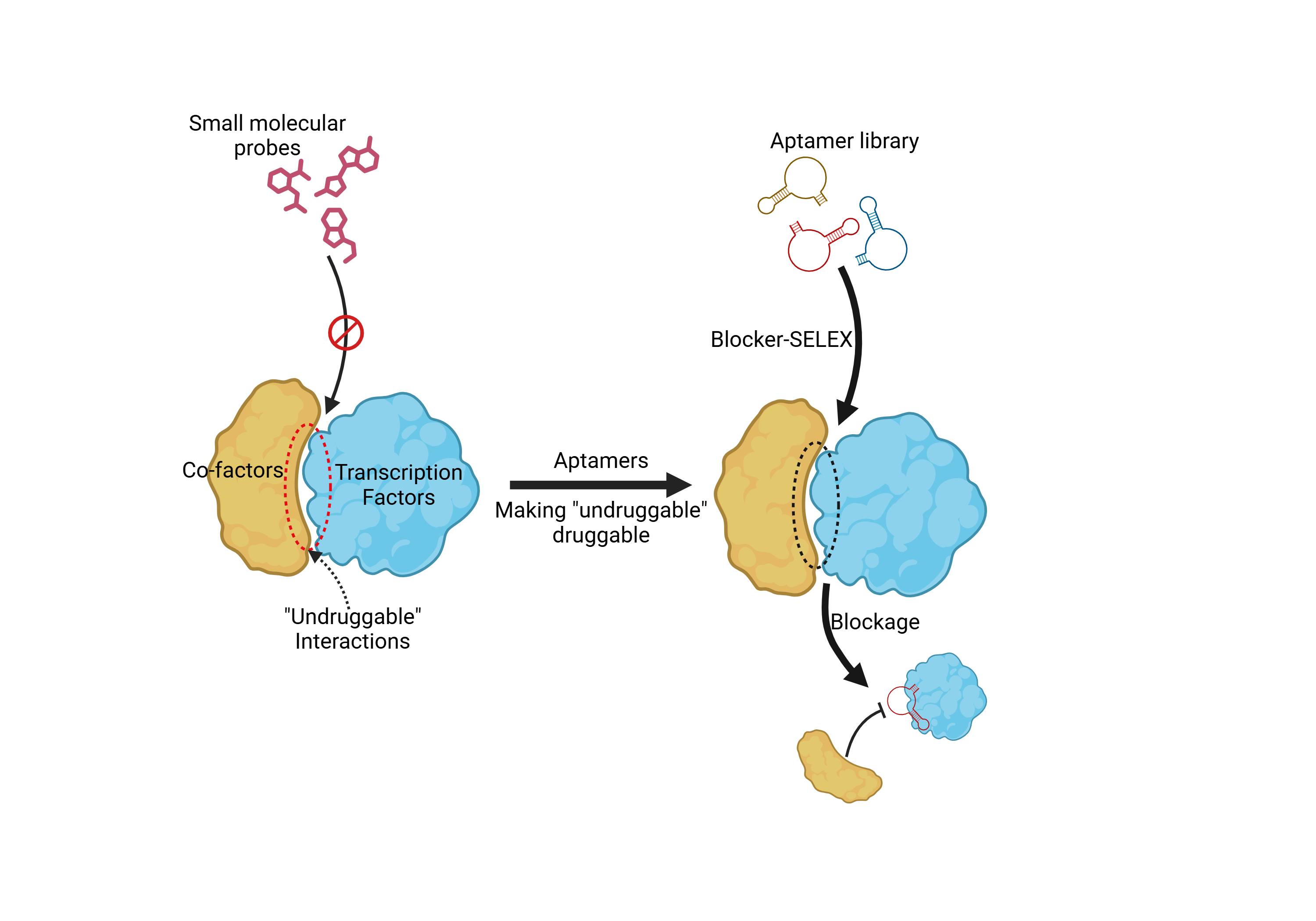
Despite the well-established significance of transcription factors (TFs) in pathogenesis, their utilization as pharmacological targets has been limited by the inherent challenges in modulating their protein interactions. The lack of defined small-molecule binding pockets and the nuclear localization of TFs do not favor the use of traditional tools. Aptamers possess large molecular weights, expansive blocking surfaces and efficient cellular internalization, making them compelling tools for modulating TF interactions.
In a recent study, the research team led by Dr. TAN Weihong, Dr. WU Qin, and Dr. WEI Yong from the Hangzhou Institute of Medicine, Chinese Academy of Sciences (CAS) report a structure-guided design strategy called Blocker-SELEX to develop inhibitory aptamers (iAptamers) that selectively block TF interactions.
This work was published in Nature Communications.
Their approach leads to the discovery of iAptamers that cooperatively disrupt SCAF4/SCAF8-RNAP2 interactions, dysregulating RNAP2-dependent gene expression, which impairs cell proliferation. This approach is further applied to develop iAptamers blocking WDR5-MYC interactions. Overall, the study highlights the potential of iAptamers in disrupting pathogenic TF interactions, implicating their potential utility in studying the biological functions of TF interactions and in nucleic acids drug discovery.
Original link: https://www.nature.com/articles/s41467-024-51197-w