Research Highlights (March–May 2025)
Cancer Cell
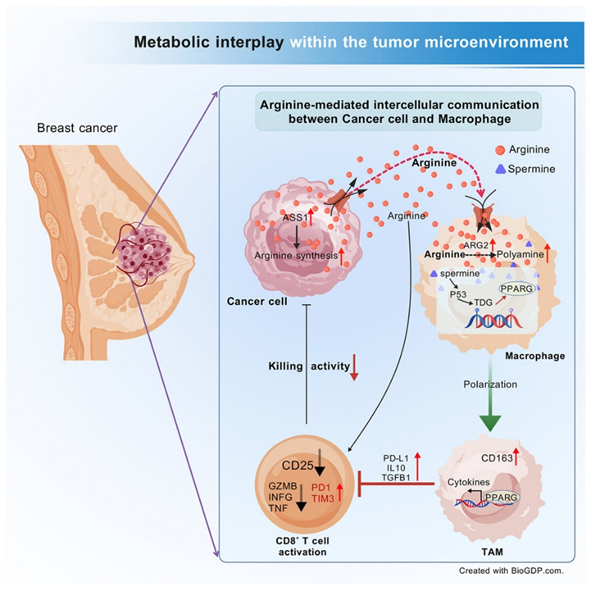
"Cancer cell-derived arginine fuels polyamine biosynthesis in tumor-associated macrophages to promote immune evasion"
Published in Cancer Cell on 10 April 2025, the study led by Dr. Hai HU revealed a novel mechanism of immune evasion in breast cancer. The research team demonstrated how tumor cells exploit arginine metabolism to produce polyamines, particularly spermine, which actively reprogram tumor-associated macrophages (TAMs) into immunosuppressive phenotypes. Through comprehensive metabolic profiling and functional assays, they identified that cancer cell-secreted arginine activates the polyamine synthesis pathway in TAMs, driving TDG-mediated DNA demethylation that ultimately suppresses CD8+ T cell function. Importantly, pharmacological targeting of key enzymes in this pathway significantly reduced tumor growth in preclinical models and restored anti-tumor immunity, offering a promising new metabolic intervention strategy for breast cancer immunotherapy.
Proceedings of the National Academy of Sciences (PNAS)
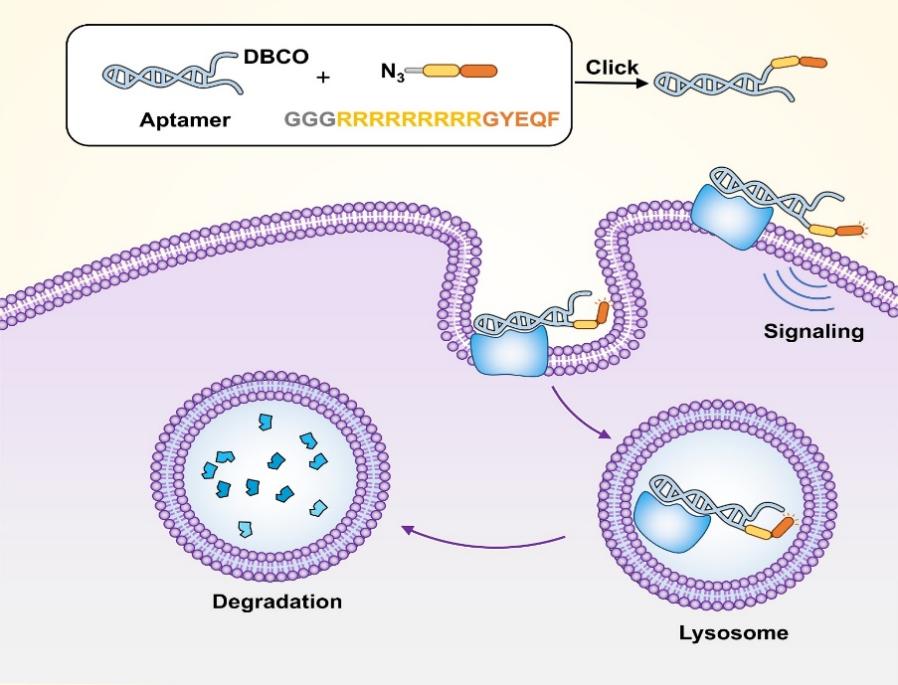
"Click-constructed Modular Signal Aptamer Chimeras Enable Receptor-independent Degradation of Membrane Proteins"
In their PNAS publication dated 2 May 2025, Drs. Fengli QU and Hui ZHANG introduced a groundbreaking protein degradation platform called Signal Aptamer Chimeras (SApts). This innovative technology combines lysosomal sorting motifs (YXXØ) with target-specific aptamers through efficient click chemistry reactions. Unlike traditional LYTACs that are limited by lysosomal receptor availability, the SApt system achieves universal degradation of various membrane proteins (including PTK7 and Met) across different cell types by hijacking endogenous endocytosis pathways. The team provided compelling evidence that SApt-mediated degradation not only removes target proteins but also significantly alters downstream signaling pathways and cellular functions, establishing this as a versatile new tool for membrane protein targeting with broad applications in cancer therapy and basic research.
Nature Communications
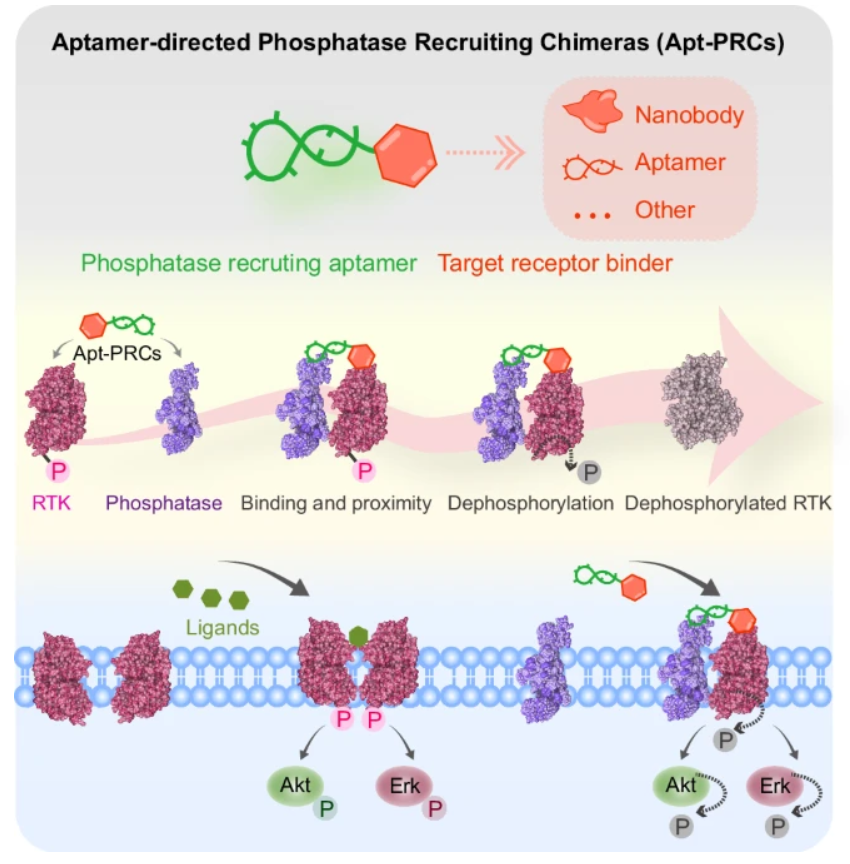
"Engineering aptamer-directed phosphatase recruiting chimeras: a strategy for modulating receptor function and overcoming drug resistance"
Dr. Juan LI's team reported their significant findings in Nature Communications on 18 April 2025, presenting a novel class of therapeutic molecules called Aptamer-Phosphatase Recruiting Chimeras (Apt-PRCs). These ingeniously designed bifunctional molecules combine the precision of receptor-targeting aptamers with the enzymatic activity of phosphatases to achieve spatially controlled dephosphorylation of oncogenic receptors like EGFR and MET. In multiple drug-resistant cancer models, the Apt-PRCs successfully restored sensitivity to tyrosine kinase inhibitors such as gefitinib and demonstrated potent tumor growth suppression in vivo. The modular nature of this platform allows for easy adaptation to target various pathological receptors, offering a generalizable new approach to disrupt aberrant signaling pathways in cancer and potentially other diseases characterized by pathological phosphorylation events.
Science Advances
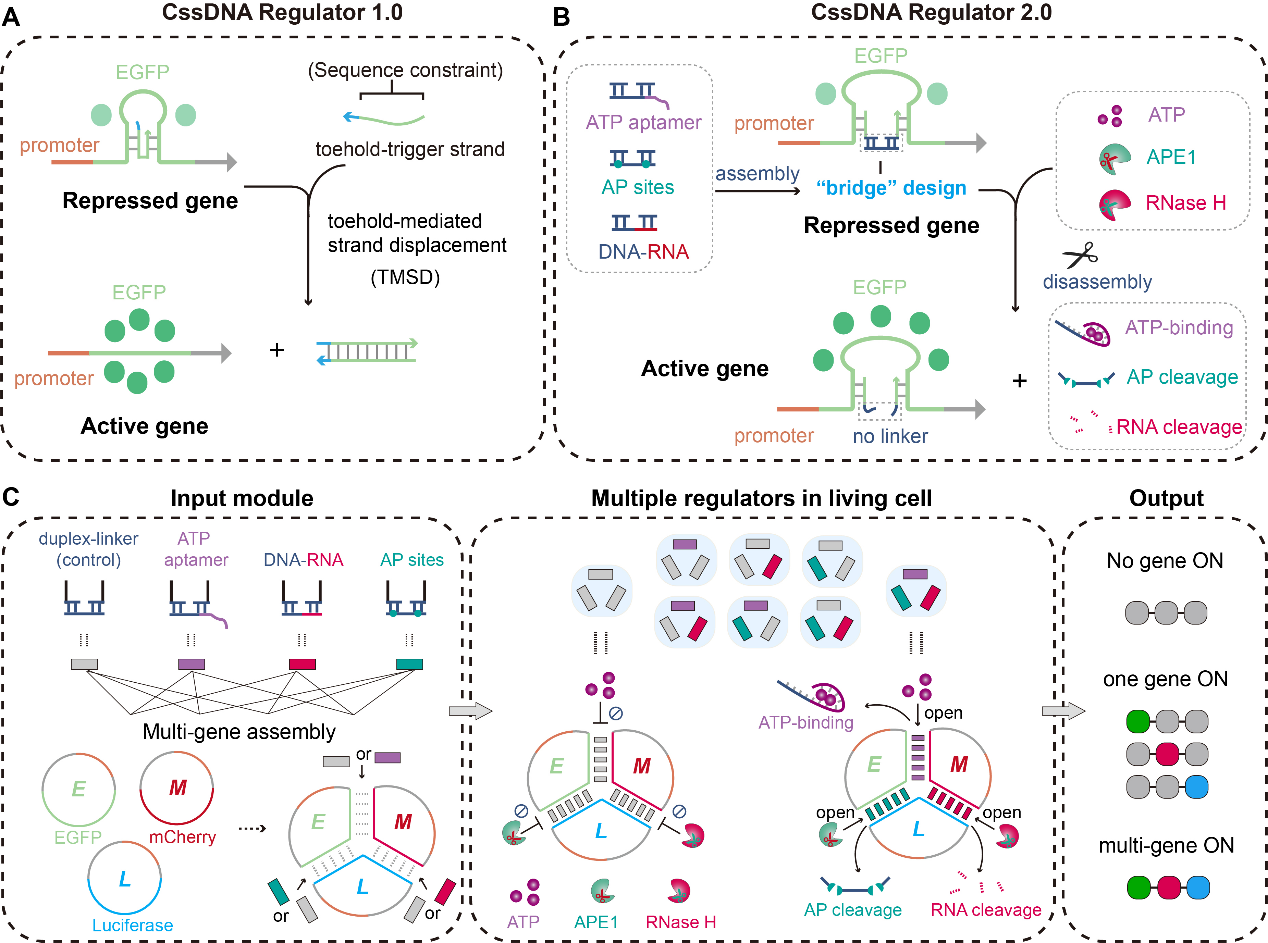
"Upgraded circular single-stranded DNA regulators for Multiple-Input-Multiple-Output gene circuits in mammalian cells"
Published in Science Advances on 15 March 2025, the work by Dr. Jie SONG's group represents a major advancement in synthetic biology tools. The team developed an enhanced version of circular single-stranded DNA (CssDNA) regulators (designated as v2.0) featuring significantly improved programmability and control capabilities. By incorporating innovative duplex bridge structures, these next-generation regulators can process multiple biological inputs (including ATP levels and APE1 activity) to drive complex Multiple-Input-Multiple-Output (MIMO) gene circuits in mammalian cells. The researchers demonstrated the system's versatility through various applications including logic-gated gene expression and precise metabolic pathway regulation, highlighting its tremendous potential for advancing synthetic biology applications, cell-based therapies, and fundamental biological research.
Journal of the American Chemical Society (JACS)
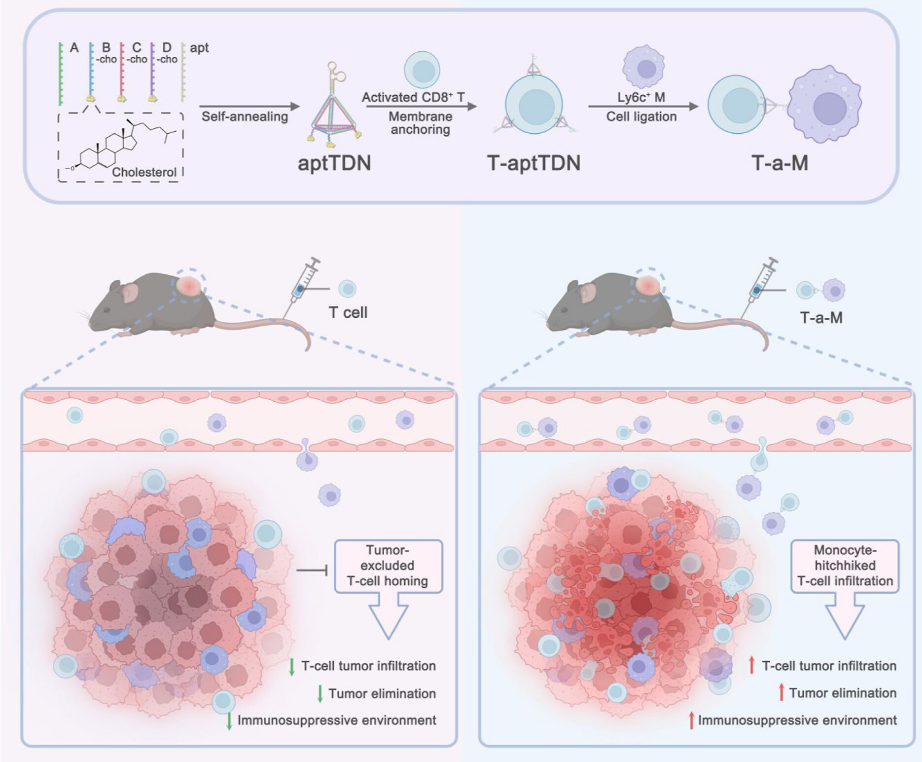
"Enhancing T-Cell Infiltration and Immunity in Solid Tumors via DNA Nanolinker-Mediated Monocyte Hitchhiking"
The Journal of the American Chemical Society featured this important work on 22 March 2025, where Dr. Liping QIU presented an innovative solution to the critical challenge of poor T cell infiltration in solid tumors. The research team designed a sophisticated DNA nanolinker called aptTDN that physically couples cytotoxic T cells to monocytes - immune cells that naturally home to tumors. This "hitchhiking" strategy cleverly exploits monocyte trafficking pathways to effectively deliver functional T cells deep into tumor cores, achieving an impressive 15-fold increase in T cell abundance in preclinical models. The approach demonstrated significant enhancement of tumor regression without causing systemic toxicity, effectively addressing one of the major limitations currently hampering the success of adoptive T cell therapies for solid tumors.
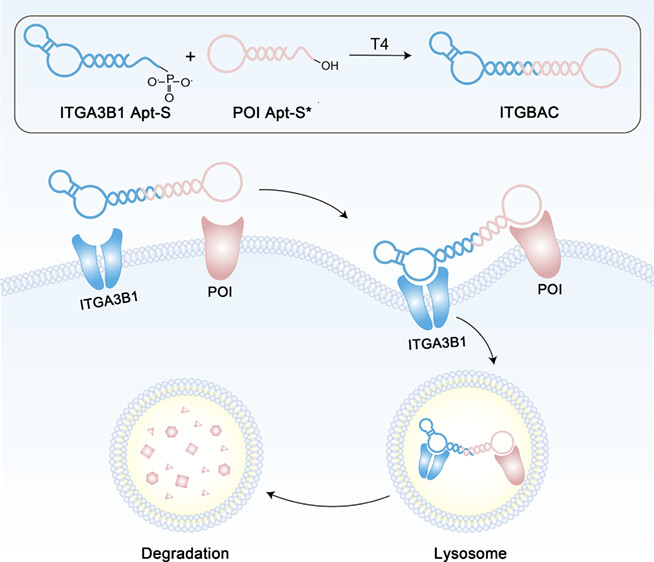
Journal of the American Chemical Society
Published on 22 March 2025 in JACS, this study by Academician Weihong TAN and Dr. Fengli QU presents bispecific aptamer chimeras (ITGBACs) that exploit integrin α3β1 for targeted CD71 degradation. The team first identified a DU145-specific aptamer (DML-7) through cell screening, then engineered chimeras that co-opt integrin-mediated internalization to shuttle CD71 to lysosomes. This approach significantly reduced off-target effects while maintaining potent cell cycle disruption and antitumor activity in vivo.
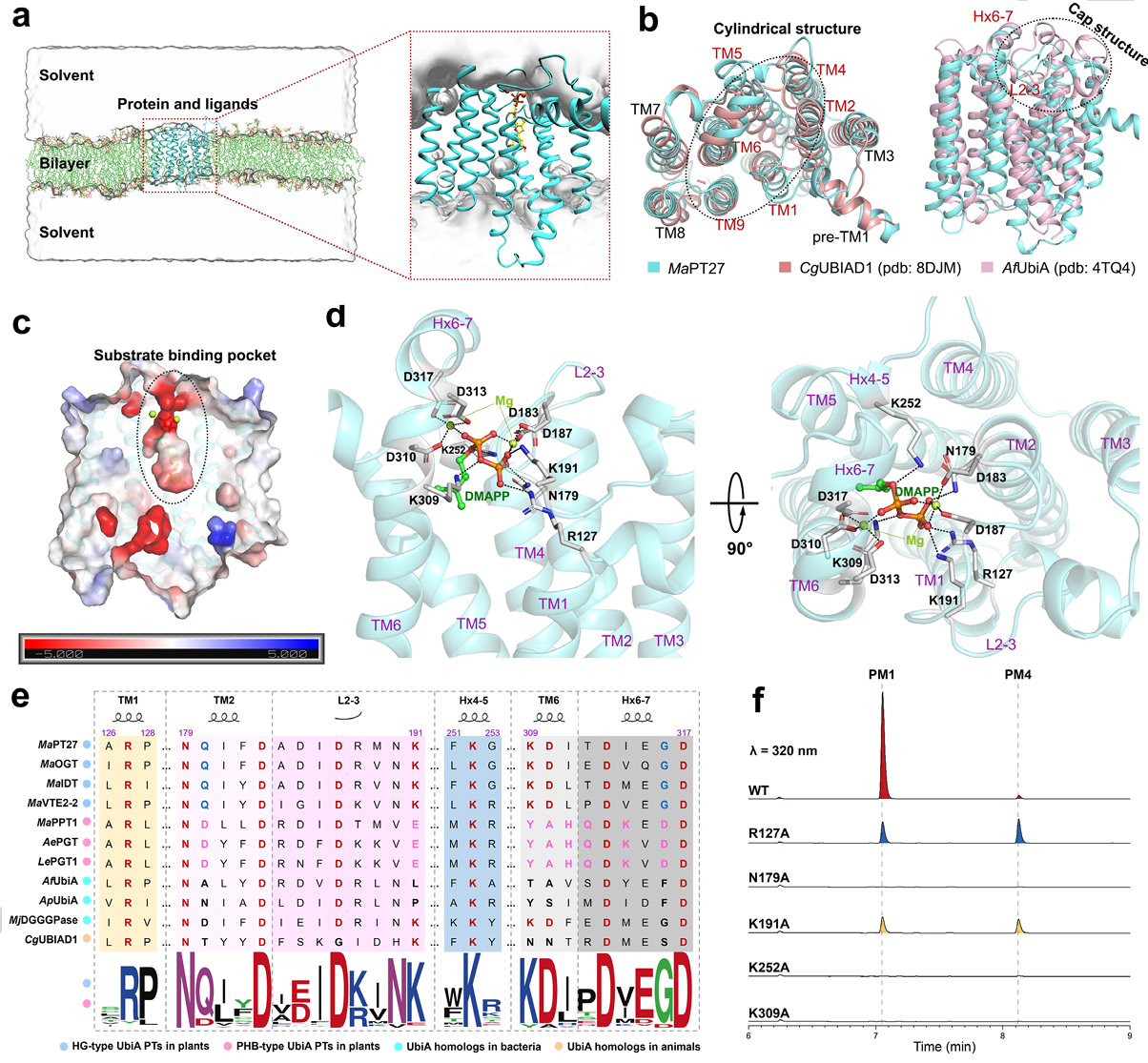
Angewandte Chemie International Edition
The 15 March 2025 issue of Angewandte Chemie features work by Drs. Jingkui TIAN and Wei ZHU elucidating prenyltransferase mechanisms in Morus alba. Through combined molecular dynamics and quantum mechanical calculations, the team characterized multiple UbiA family enzymes that install prenyl groups at specific positions on morusin scaffolds. Their findings reveal how both substrate chemistry and active site architecture jointly determine regioselectivity in these medicinally important modifications.
Angewandte Chemie International Edition
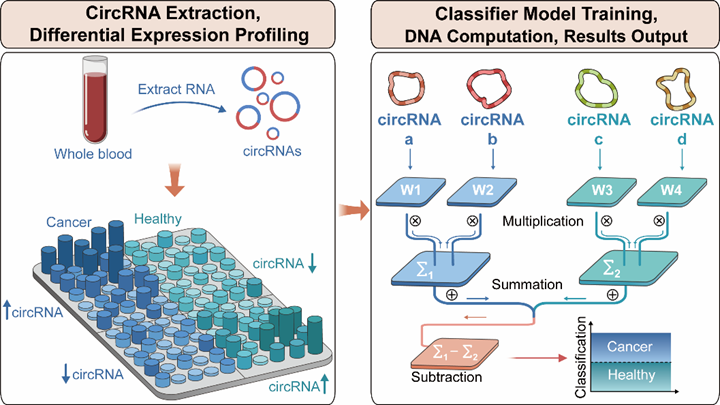
"Circular RNA-Based Molecular Computation Enhances Plasma Biomarker Detection in Biliary Tract Cancer"
Published 10 April 2025 in Angewandte Chemie, Dr. Da HAN's team created a DNA molecular computing system that leverages circRNA stability for biliary tract cancer diagnosis. Their platform achieved 92% diagnostic accuracy in clinical samples while reducing costs by 70% compared to conventional methods, demonstrating how nucleic acid chemistry can overcome challenges in liquid biopsy development for this aggressive cancer type.
Angewandte Chemie International Edition
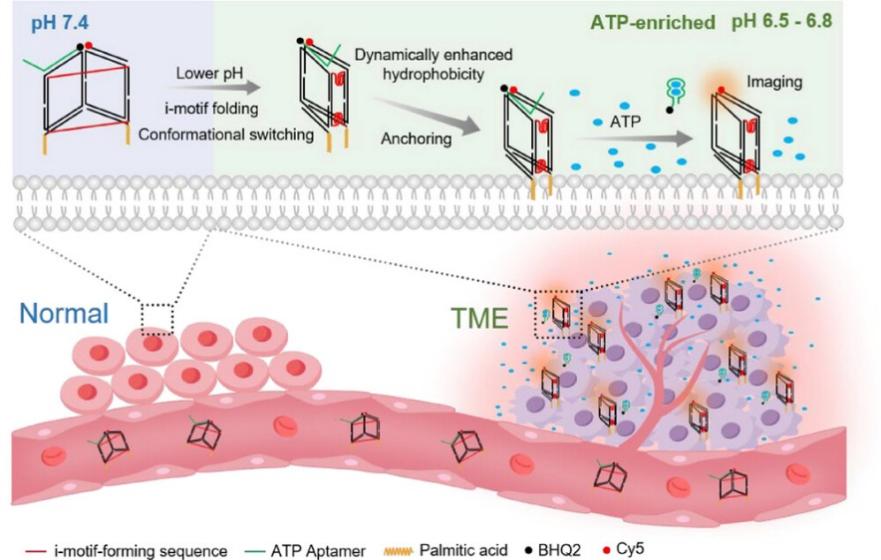
"Dynamic Modulation of Interactions Between Hydrophobic Tag-Conjugated DNA Nanoprobes and Cell Membrane Facilitates ATP Imaging in the Tumor Microenvironment"
In their Angewandte Chemie publication dated 5 April 2025, Drs. Fengli QU and Jin LI introduced a new class of pH-responsive DNA nanoprobes (HT-DNPs) that enable precise monitoring of tumor metabolism. These innovative probes utilize cleverly designed i-motif structures to control the clustering of hydrophobic tags, allowing selective anchoring to cancer cell membranes specifically in acidic tumor microenvironments (pH 6.5-6.8). The technology provides researchers with an unprecedented ability to perform spatial-specific imaging of ATP distribution and dynamics within tumors through a fluorescence activation mechanism. This breakthrough not only offers new insights into metabolic crosstalk within the tumor microenvironment but also has significant potential applications in cancer diagnostics, therapy monitoring, and fundamental studies of tumor metabolism.
Advanced Materials
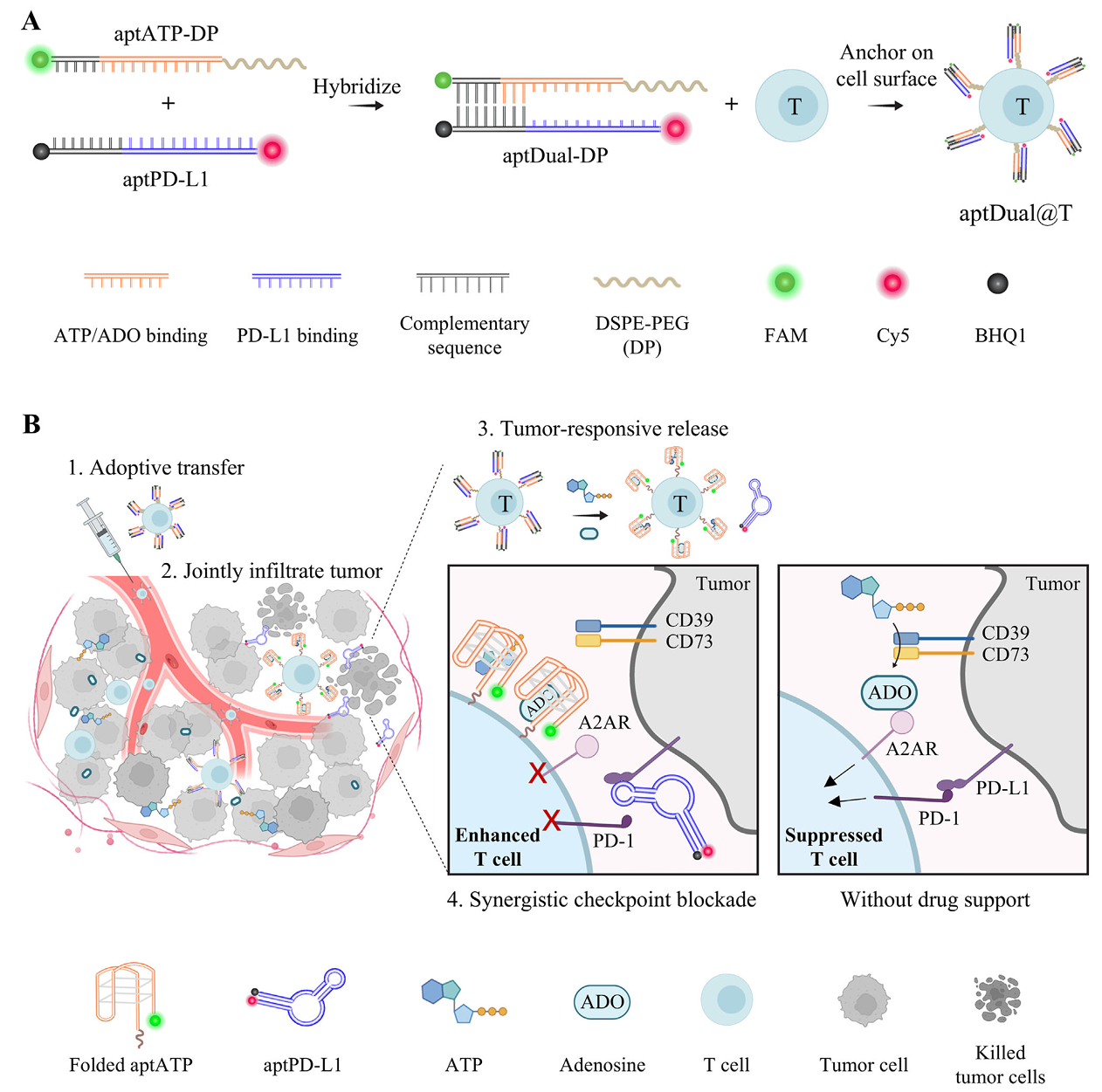
"Cell Surface-tethered Nucleic Acid Therapeutics Program Robust and Tumor-responsive Enhancement of Adoptive Cell Therapy"
Published in Advanced Materials on 12 May 2025, the collaborative work by Drs. Yajun WANG and Yiran ZHENG represents a major leap forward in cell therapy engineering. The team developed sophisticated "nucleic acid backpacks" that can be securely anchored to CAR-T cells and programmed to release immune checkpoint inhibitors (targeting both PD-1 and adenosine pathways) specifically in response to tumor microenvironmental cues. This innovative approach achieved remarkable results, increasing tumor-infiltrating CAR-T cell numbers by 40-fold and reaching 60% cure rates in aggressive solid tumor models, all while minimizing off-target effects that commonly plague systemic immunotherapy approaches. The technology establishes a new paradigm for enhancing adoptive cell therapies through precisely controlled, localized drug delivery.
Nucleic Acids Research
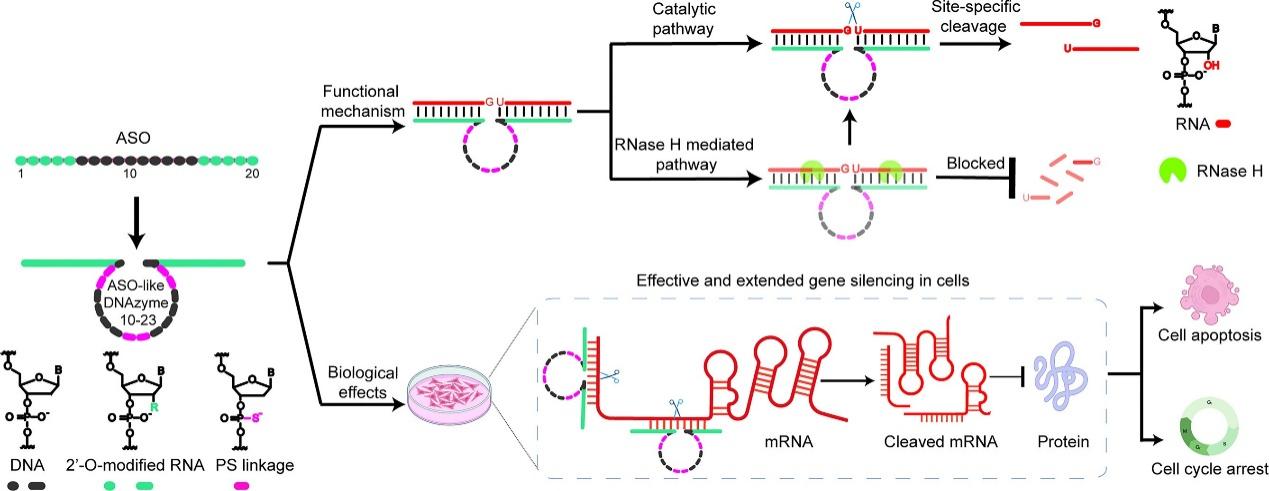
"Chemical evolution of ASO-like DNAzymes for effective and extended gene silencing in cells"
The 5 March 2025 Nucleic Acids Research publication by Dr. Yajun WANG describes engineered DNAzymes that combine the best features of ASOs and catalytic nucleic acids. By incorporating 2'-O-methyl and phosphorothioate modifications, these 10-23 DNAzyme variants achieved 85% knockdown of oncogenic c-MYC in colon cancer cells with 3-fold fewer off-target effects than conventional constructs, as verified by RACE-PCR and RNA-seq analyses.
Cancer Discovery
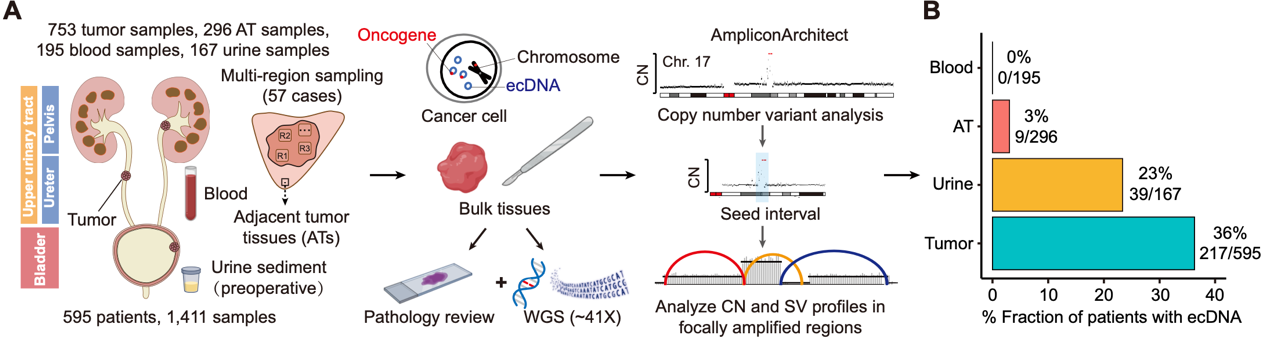
"Spatial-Temporal Diversity of Extrachromosomal DNA Shapes Urothelial Carcinoma Evolution and Tumor-Immune Microenvironment"
The Cancer Discovery paper published on 28 March 2025 presents groundbreaking insights from a multi-institutional study on extrachromosomal DNA (ecDNA) in urothelial cancer. Through comprehensive analysis of the CUGA-595 genomic cohort, the research team meticulously mapped how ecDNA drives both clonal expansion and immune evasion mechanisms in this challenging cancer type. The study revealed strong correlations between ecDNA structural diversity and poor clinical prognosis, while also identifying its crucial role in suppressing cytotoxic immune responses. A particularly significant translational outcome was the development of a noninvasive urine test that detects ecDNA with 100% specificity, offering clinicians a powerful new tool for early cancer detection and intervention.
Advanced Science
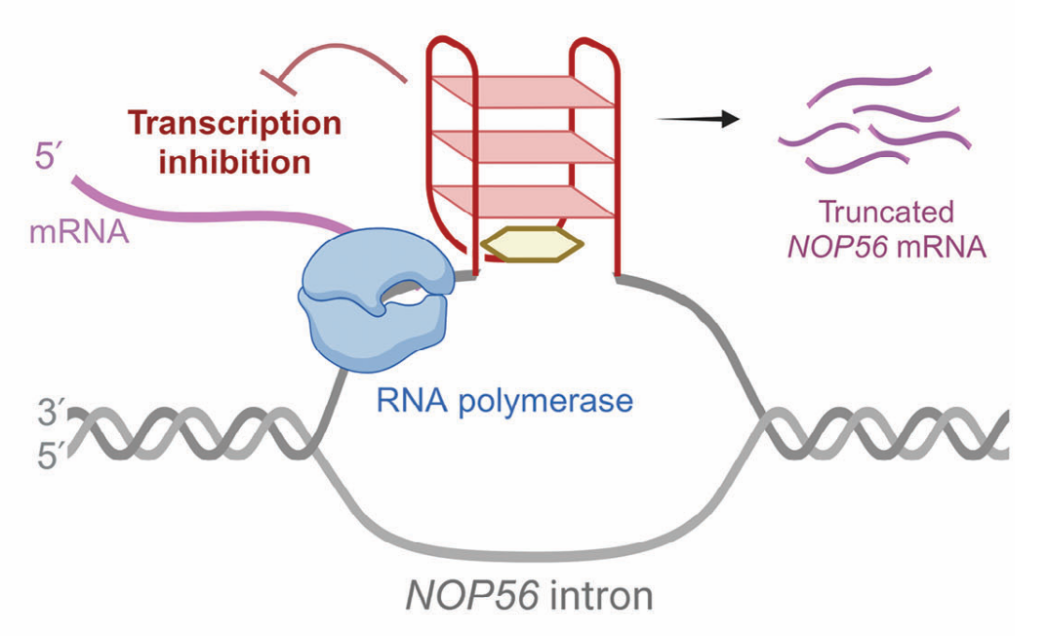
"Structural Insights into an Antiparallel Chair-Type G-Quadruplex From the Intron of NOP56 Oncogene"
Drs. Da HAN and Pei GUO's Advanced Science publication on 22 April 2025 reported the successful determination of the NMR structure of a unique G-quadruplex (NOP56-G4) located in the intronic region of the NOP56 oncogene. The researchers revealed an unusual antiparallel "chair" topology that distinguishes this structure from previously characterized G-quadruplexes. Functional studies demonstrated that stabilizing NOP56-G4 with the small molecule PDS effectively reduced oncogene expression in cancer cells, providing compelling validation of this structure as a druggable target. This finding is particularly significant for KRAS-mutant cancers, opening new avenues for targeted intervention in these traditionally challenging-to-treat malignancies.
Advanced Science
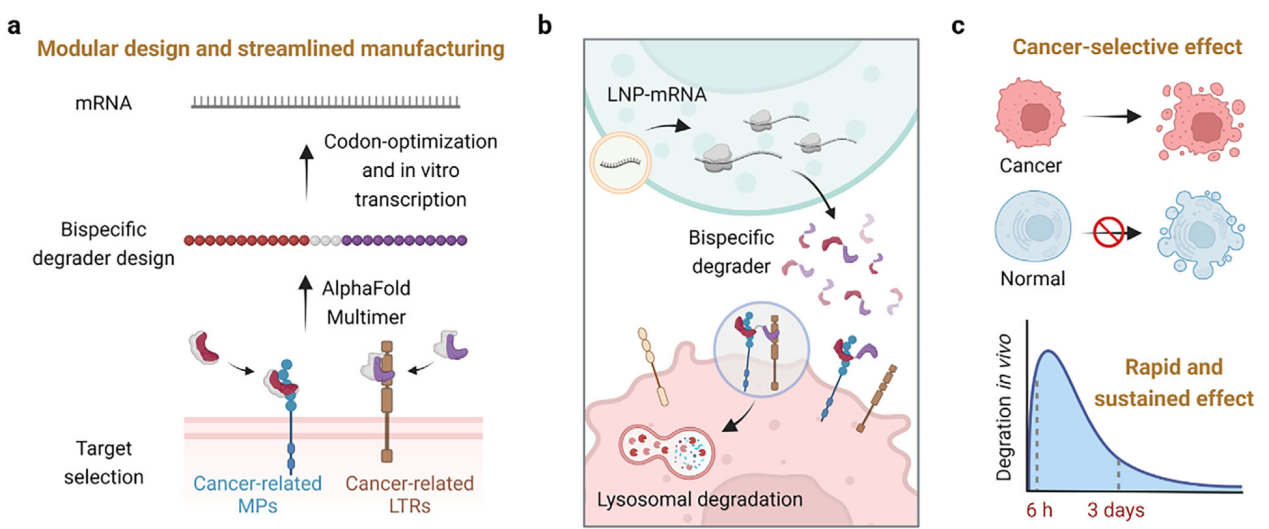
"Sortilin-Mediated Rapid, Precise and Sustained Degradation of Membrane Proteins via mRNA-Encoded Lysosome-Targeting Chimera"
In their 18 April 2025 Advanced Science paper, Academician Weihong TAN and Dr. ZUO introduced mRNA-encoded MedTACs - an innovative platform for membrane protein degradation. Using computationally designed sortilin-binding scFvs, their system mediated rapid (t1/2 <2h), sustained (>72h) degradation of oncogenic membrane proteins while overcoming production challenges of recombinant protein degraders.
Cancer Research
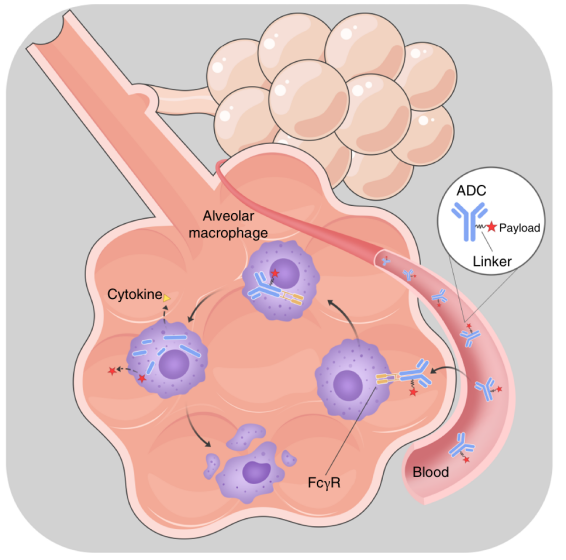
"Perivascular Niche-Resident Alveolar Macrophages Promote Interstitial Pneumonitis Related to Trastuzumab Deruxtecan Treatment"
Published 12 April 2025 in Cancer Research, Dr. Peng GUO's study identified the cellular mechanism behind T-DXd-induced lung toxicity. Their "ADC+A" pretreatment strategy reduced pneumonitis incidence from 18% to 3% in models while maintaining antitumor efficacy - critical findings for improving the safety profile of this important class of anticancer drugs.
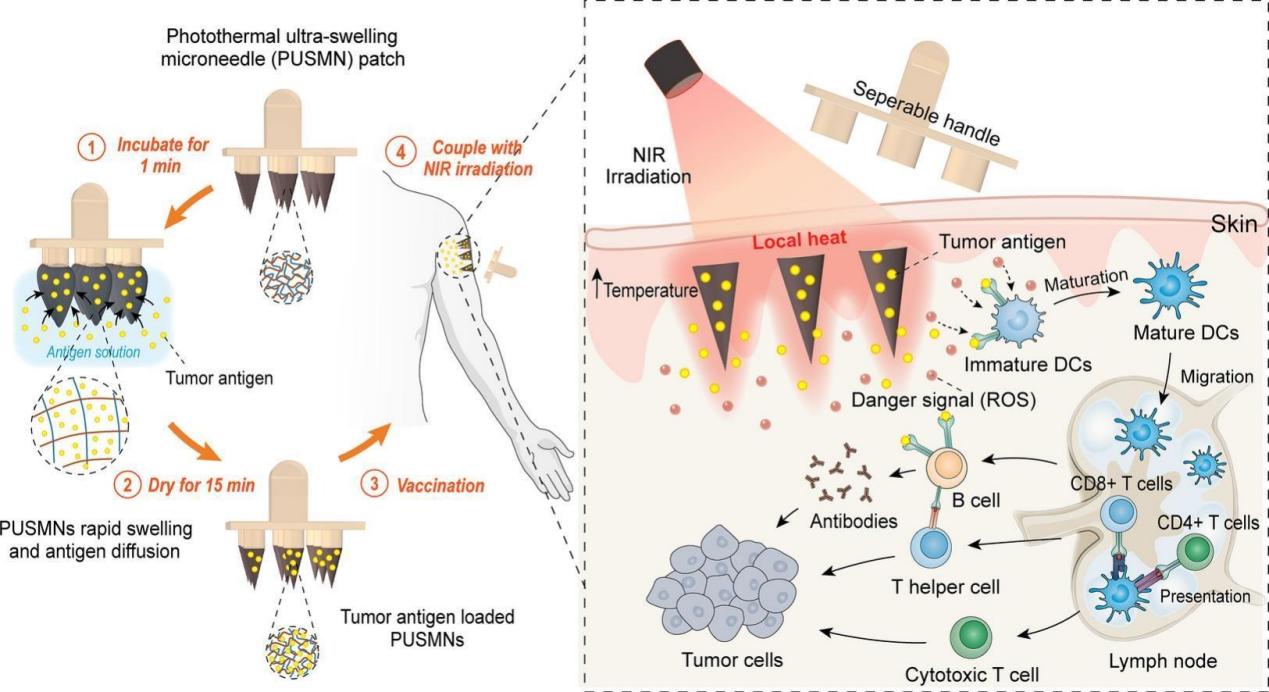
Journal of Controlled Release
"Ice-pop making inspired photothermal ultra-swelling microneedles to facilitate loading and intradermal vaccination of tumor antigen"
The 8 March 2025 Journal of Controlled Release featured Dr. Hao CHANG's innovative PUSMN microneedle platform. Inspired by popsicle manufacturing, these needles achieve 2000% expansion for rapid antigen loading and, when combined with NIR activation, induce 5-fold stronger T cell responses than conventional delivery methods - a significant advance for cancer vaccination strategies.
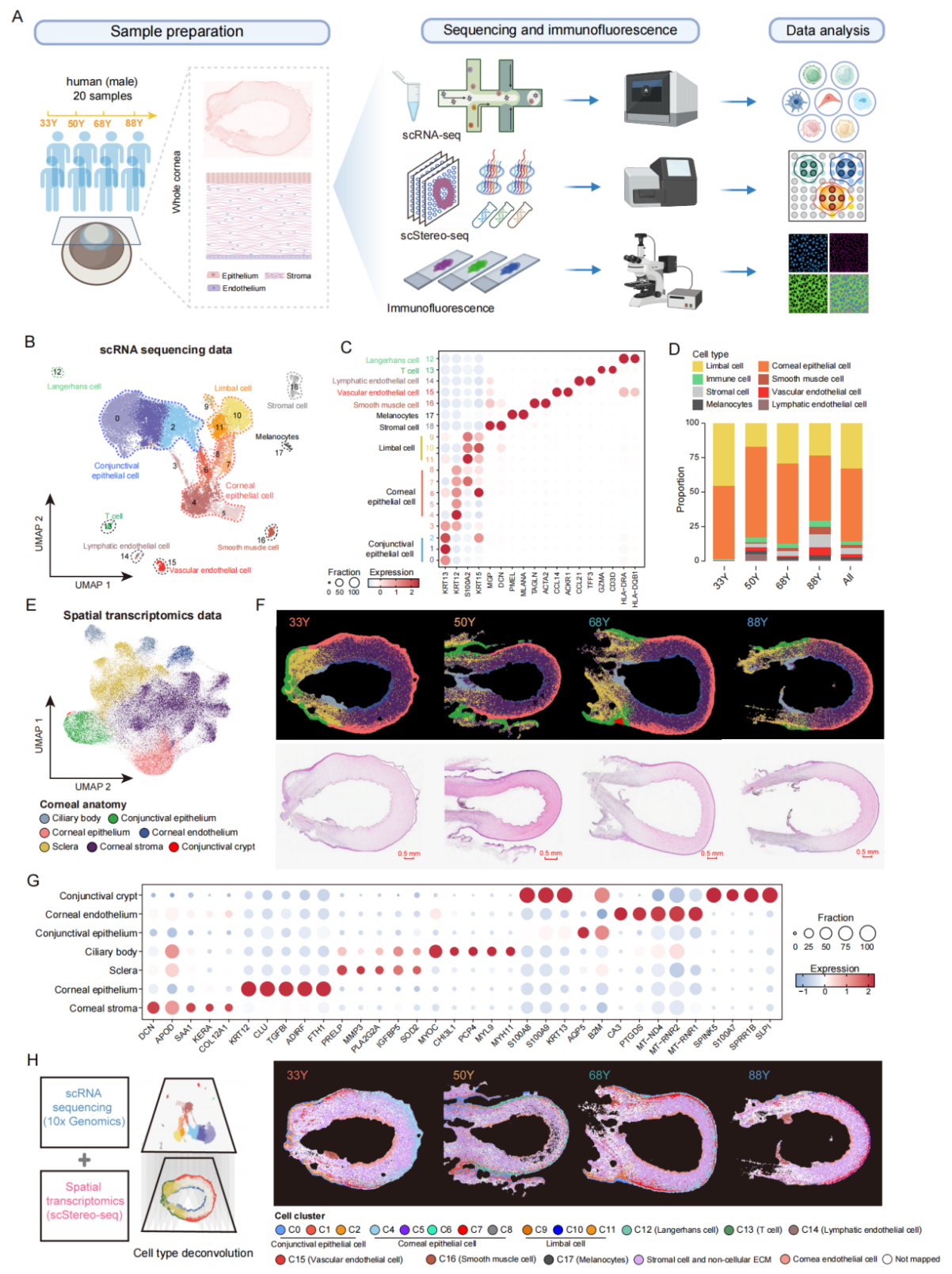
Genome Medicine
"Spatiotemporal single-cell analysis elucidates the cellular and molecular dynamics of human cornea aging"
Published in Genome Medicine on 20 May 2025, this landmark study established the first comprehensive atlas of human corneal aging through sophisticated integration of single-cell RNA sequencing and spatial transcriptomics technologies. The research team identified three key pathological drivers of age-related corneal degeneration: stem cell exhaustion, characteristic metabolic shifts, and progressive immune cell infiltration. These findings provide crucial new biomarkers for early detection of corneal aging and identify promising molecular targets for regenerative therapies. The dataset represents an invaluable resource for vision research, offering unprecedented resolution into the cellular and molecular changes underlying corneal aging processes that could lead to new therapeutic strategies for age-related eye diseases.